Radiation Hazards
The decay of an atomic nucleus produces a number of different particles which together are lumped under the designation of radiation. Radiation is measured in various ways, by detecting it's energy; but not all radiation is created equal. There are essentially four types of radiation given off, during the nuclear process. There is alpha, beta, and gamma radiation, as well as Neutrons, X-rays, heat, and considerable amounts of ordinary light.
Alpha particles are the heaviest, and least dangerous form of radiation, as far as exposure hazards are concerned. An alpha particle is essentially a helium nucleus, consisting of a pair of neutrons, and a pair of protons. It carries a positive charge, and wants desperately to pick up a pair of electrons and make itself whole. A few feet of air, will prevent penetration by alpha particles. Even so, because of their heavy mass, and double positive charge, Alpha particles actually have the highest potential for causing damage, should they be able to enter the body. Generally, Alpha particles are dangerous, only if a subject should ingest a radioactive substance which gives them off. Another positively charged particle given off, though rarely, is the proton. A proton can be thought of as a hydrogen nucleus. Like the alpha particle, the goal of the proton is to pick up an electron, in order to make itself a whole and neutral atom. Like the alpha particle, the proton rarely gets more than a few inches from it's source, before being neutralized by the surrounding air. Another charged particle given off, is the beta particle, essentially a high speed electron, or less commonly a high speed positron. Having a charge, the beta particle is seeking an opposite charge with which to make itself whole. Beta particles are stopped by ten feet or so of air, a thin sheet of metal or plastic, or by your skin.
The more dangerous forms or radiation, over distance, are those whose particles carry no charge. These are much more resistant to being neutralized, and can carry substantial amounts of energy. X-rays, and gamma rays are both forms of high energy photons, making them essentially high energy, and high frequency forms of light. Concrete, and even lead is required to stop high energy photons. These are both forms of ionizing radiation; in essence, they burn us. Though they work at the atomic level rather than the chemical level, they have the same effect on our bodies as the hydroxide (OH), which is the ultimate source of free radical chemicals in the body.
X-rays have more energy than ultra violet light; but generally not as much as gamma rays. As a general rule, gamma rays have more energy and a higher frequency than X-rays; but there is some overlap. While X-rays, and gamma rays of the same frequency are essentially the same, they are differentiated by their source. X-rays come from electrons which wiggle or bounce, while gamma rays come from a nucleus whose particles are made to wiggle or bounce. This would seem to imply that the nucleus, far from being a static collection of particles, has some internal motion; but that is a subject for another time. It is very rare for electrons to produce X-rays with so much energy that they rise into the gamma ray range, and equally rare for a nucleus to produce gamma rays at such low energy that they drop into the range of X-rays; but it does sometimes happen. Photons are pure energy, and have no mass, as well as no charge.
Probably the most
dangerous form of radiation, over distance, is the neutron.
This particle combines the
most dangerous features of all the other forms of radiation.
Neutrons, like protons, alpha particles, and beta particles,
have mass. What makes them so dangerous is that, like gamma
rays, they have no charge, and are thus not amenable to
neutralization. Neutrons do not merely ionize atoms, they
have the capability of breaking them up. Breaking up an
atom, not only destroys or transmutes it, but also releases
further radiation, including perhaps further neutrons. The
controversial neutron bomb of the seventies and eighties,
worked by enhancing the amount of neutron radiation given
off, even though this decreased the explosive yield of the
bomb. The increased neutron radiation was able to penetrate
the armor of tanks, and kill the men inside, even from miles
away. A free neutron has a half life of 10.3 minutes. It
decays into a proton, electron, and anti neutrino.
Types of radiation
Alpha An alpha particle is a helium nucleus. It consists of two neutrons, and two protons
Beta A beta particle is a high speed electron or positron
Gamma A gamma particle, or gamma ray, is an extremely high energy photon, released by the nucleus.
X-ray An X-ray is a very high energy photon, released by the electron.
Neutrons A neutron is a neutrally charged particle.
Proton A proton is a positively charged particle.
Lethal effects
Basically, Alpha and Beta
particles
do us no harm, as long as they are not ingested, or inhaled: they are stopped by our skin. X-rays, gamma radiation,
and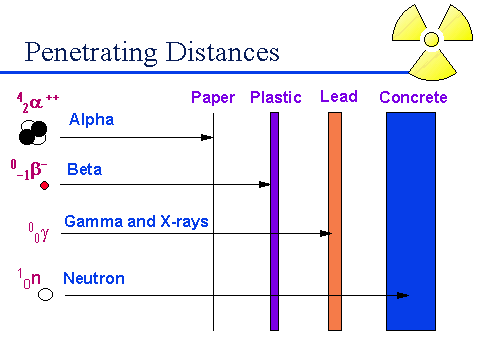 neutrons are different. X-rays and gamma radiation are both
essentially highly energetic forms of light. They harm us, as is
mentioned above, by burning us, and giving us a sort of a high energy,
and completely penetrating sunburn. At high enough levels, these effects
are lethal, due to their ability to damage the repair and reproductive
abilities at the cellular level.
neutrons are different. X-rays and gamma radiation are both
essentially highly energetic forms of light. They harm us, as is
mentioned above, by burning us, and giving us a sort of a high energy,
and completely penetrating sunburn. At high enough levels, these effects
are lethal, due to their ability to damage the repair and reproductive
abilities at the cellular level.
Still, though there is some direct damage, via radiation, particularly with neutrons, much of the ultimate damage done to us by exposure is chemical. The effects are largely cellular, rather than systemic. Systems are affected only so far as the cells by which they are made up are damaged. This knowledge makes some of the more mysterious symptoms of radiation poisoning a bit easier to understand. It also gives a mechanism for the apparent remission of symptoms, soon after exposure, only to return in far more severe form latter on. The main, short term effect, of radiation is ionization. Ionization makes the substances within the cell much less stable, and has a tendency to split compounds up into unstable compounds commonly called free radicals.
Free radicals are the terror of the modern health faddist, and are implicated in the aging process, as well as numerous diseases, chronic conditions, and bodily malfunctions. To a certain extent their production is a normal side effect of cellular processes, and much of the mechanism of the cell is dedicated to their neutralization. Ionizing radiation has enough energy to remove electrons from the atoms that make up molecules of cellular components. The most likely electrons to be removed are the shared electrons, which make up chemical bonds holding molecules together, since these are the most lightly held. When a shared electron is dislodged by ionizing radiation, the bond it creates is broken and, the molecule falls apart. In addition, the energy contained by the radiation is transferred to the cell, adding to its heat. In simple materials, like most metals, and gases, ionization is not a big deal. The negative electrons soon rejoin the positive ionized particles, and everything gets back to normal. The more complicated the chemical structure, the more disrupted it can become. Living structures are as complicated as they come, and are seriously affected by ionizing radiation.
Ionization of cellular materials produces a number of different free radicals; but since water is by far the most abundant material within the cell, it comprises the largest number of molecules ionized. When water is so ionized, it releases free electrons, and breaks apart into charged H, and OH particles, which are very reactive, and may combine unpredictably with other substances to damage the cell. The free electrons released, have a chemical symbol of e-aq, and are the strongest reducing agents known, with pKa = 9.7, having a reduction potential Eo' = - 2.9 V at pH 7. These free electrons combine to make O2- in oxygenated systems. This is a strong oxidizing agent and the precursor of hydrogen peroxide, which is itself a powerful free radical, and serious poison. The fate of the other primary radiolysis products has been deduced from indirect evidence. H2O+ ions are unstable and decompose within 10-13 seconds to form OH· radicals by transferring a proton to an adjacent H2O molecule. It should also be noted that every change creates residues, and releases energy, to further damage the cell.
What this adds up to is a huge, disorganized, unmanageable mess, within the cell. Now cells are designed to deal with free radicals, as these are a natural product of many of the oxidation processes which take place within the cell organelles; but they are not designed to deal with such a large concentration, nor are they designed to deal with such a random distribution of these substances throughout the cell. Generally, free radicals are concentrated in specific reaction areas or organelles, where they are contained and managed. Unlike free radical production due to the chemical activities of the body, free radical production through radiation exposure is fairly random, both in the chemicals produced, and their location within the cell. This makes them somewhat less amenable to normal cellular processes of neutralization.
The amounts of these substances produced are related to the dose absorbed. In turn, the effects on the cells, and on the body as a whole, are dependant upon the amounts of these chemicals in the cells. Some cells are more resistant, or more accurately they are better able to repair themselves. As a general rule, the most vulnerable cells are those with higher metabolisms, and lower specialization.
Though all cells are damaged more or less equally, by equal amounts of radiation, cells with higher metabolisms, higher rates of reproduction, or less specialized functions have less time to recover, and thus are affected more strongly. This is largely because these types of cells are more more active and highly stressed, with less reserve, and less margin for error. These cells place more of a burden on their various structures, but particularly upon the DNA. As an example, a cell which divides rapidly, will likely be called upon to divide before chromosomal damage can be repaired, and thus die in the attempt, or possibly divide improperly, even to the extent that it may become cancerous. A less active cell, may not be required to call upon it's DNA so soon, and thus give it's chromosomes time enough to repair themselves. Higher exposures cause more damage, taking more time to repair, which in turn overtly affects more cell types, and can lead to noticeable impairment of increasingly resistant bodily systems. Eventually, a point is reached where chromosomal damage is so severe, that repair is impossible, no matter how long the cell is given. Note that cells may be damaged, at certain levels of exposure, without any overt expression of radiation sickness showing.
When cellular damage reaches the point at which noticeable symptoms manifest themselves, the condition is called acute radiation syndrome, also know as radiation sickness, or sometimes as radiation poisoning. All three terms refer to the same thing. This is the threshold at which damage affects cell function, or causes cell death, to the extent that the functioning of bodily systems, and of the whole body itself, becomes impaired. Effects are variable, depending upon the random distribution of the radioactive particles, and the state of being of the subject. There are also certain things that just can not be determined ahead of time, like the particular cells and particular parts of cells, struck by radioactive particles. Because of this, lethality of a given does is expressed statistically as a function of the odds of death over time. This is striking in it's similarity to the method of determining radioactive decay in fissionables, by their half lives.
Radiation Energy, and Units of Measure
A Curie is a unit of measurement based upon the amount of radiation produced by one gram of radium. In this case, 37 billion atoms fission each second. This archaic unit of measurement was adopted because in the early days of nuclear research, radium was the only readily available fissionable, and the true nature of radiation was not clearly known. A more modern unit of measurement, based upon a better understanding of nuclear radiation is the Becquerel. This is simply the number of fissions per second, making 37 billion Becquerels equal to one curie. So what do these numbers tell us? Well, for bomb makers, and reactor designers, they are significant, indicating the specific activity of a particular substance. For medical professionals, and unfortunate victims exposed to radiation, they don't tell us much at all. What was needed was a different sort of measurement, and a way to gauge the effect of radiation on the body. A raw measurement of radioactivity will no more indicate lethality, than a raw measurement of horsepower will indicate how fast a car might go, or how much it might carry.
The lethal dose of Radiation has come to be defined by what is called the LD 50/30. This is a shorthand way of saying that this is the dose which will kill 50% of those exposed, within 30 days of the initial exposure. In the average human, this has been determined to be a dose of 450 RAD, though the range goes from 300 to 500 RAD. This would probably be a good time to detail how radiation dose is defined, and the various measurement systems used, since several different units are used, and their relationships can be as confusing and interrelated as those in a soap opera. The confusion comes, because there are three current sets of measurements (and one old set), which describe three different things - radiation traveling through the air, dose absorbed by tissue, and effective dose dependent upon radiation type. The units of measurement are the Roentgen, RAD, Gray, REM, and Sievert.
So here’s how it works. The unit of exposure, is the Roentgen. This is the raw unit of radiation energy. This is the amount of radiation traveling through the air, which will cause ionization to the extent of 1 electrostatic unit per CC of the air through which it passes. Though this is an accurate measure of pure radiation energy transmitted, it is only casually related to the amount of effect that may be transmitted to the body. Effect on the body, has traditionally been indicated by the RAD.
One RAD is equal to 100 ergs of energy, absorbed per gram of body weight. A newer measurement is the gray (Gy), which is equal to 100 RAD, and happens to be equal to 1 joule of energy absorbed per kilo of body weight. Even this, though, does not tell the whole story because, as was alluded to towards the top of the page, not all radiation is created equal. In order to compensate for the differing effects of the different types of radiation, a unit of measurement called the REM was created.
One REM uses the damage caused by gamma radiation, as a baseline, and applies a compensation factor to properly represent the effects of the other radiation types. To determine the amount of REMs absorbed by the body, the number of RADS absorbed by the body is multiplied by the following factors.
X-rays, Gamma rays, Beta particles, use a multiplier of 1, so one RAD of any of these types of radiation is equal to one REM.
Slow Neutrons use a multiplier of 3, so one RAD of slow neutrons is equal to three REM.
Fast Neutrons use a multiplier of 10.
Protons use a multiplier of 10.
Alpha Particles use a multiplier of 20.
The Sievert (Sv) is a more current version of the REM, and is based upon the Gray instead of the RAD. It is thus equal to 100 REM.
Acute Radiation Syndrome
There are many environmental agents, which attack our bodies, including bacteria, various organic and inorganic poisons, and even the rays of the sun, which at high levels can give us sunburn, or cancer. Among these agents, is a certain amount of background radiation. Throughout our lives our cells are constantly being exposed to background radiation, which does a certain amount of damage. This occurs every day, and is a part of the normal wear and tear that our bodies endure, from all sources, and to which they have adapted repair mechanisms. A dose beyond what is normal is referred to as an acute dose, implying that this is a unique, or one time event, something out of the ordinary. This is the dose at which the normal mechanisms of repair are no longer adequate to maintain normal cellular or bodily function. At the cellular level, acute radiation damage appears at doses as low as 20 REM, with some temporary, and slight lowering of red blood count showing at levels of between 20 - 50 REM. At Between 50 - 100 REM, there may be some signs of distress, such as nausea, headache or infection, the so called flu-like symptoms. Because these symptoms are not unique to radiation poisoning, and generally do not recur, this is not generally considered to be radiation syndrome until a dose of at least 200 REM is received.
In actual Acute Radiation Syndrome, there are a couple of different stages, with a period of remission in between. The first sign of acute radiation syndrome is generally nausea, vomiting, headache, and weakness. This is called the prodromal stage. The onset of this first wave of sickness can occur immediately after exposure, for very high doses, or may not become apparent for several hours, or even as long as a day or two, again depending upon the dose received. The exact cause of this first stage is not entirely understood. It appears to be a sort of a shock, lasting until the body can marshal it's reserves, and adapt itself to the damage inflicted. This causes a period of remission. During the latent stage, the victim may feel as if recovery has been made. This stage can last anywhere from a few days to two weeks, depending upon dosage. At very high dosages, the latent stage may last only a few hours. The latent stage ends, when the body uses up it's reserves, and the individual cells are once again required to function normally. If the cells have not been able to repair damage by this time, the illness stage begins. This is also about the time, when the inability of certain cells to reproduce and replace normal attrition begins to show. This is particularly the case for the production of red blood cells, and for the cells lining the GI track, which have a normal lifespan of just a few days.
Following the latent stage is the stage of actual illness. This stage can last from two to six weeks, in the case of survival, or will continue until death of the subject in a shorter period of time. Generally, if the victim emerges from the illness phase, or survives past six weeks, the illness will have run it's course. This does not mean that a full recovery has been made, or will ever be made. In many cases, particularly at higher doses, cancer, or other complications are likely to follow in subsequent years.
The hallmark of radiation exposure, and the cause of many of the symptoms, is chromosome damage. This can occur in a couple of different ways. Chromosomes may be damaged directly, through direct ionization by radioactive particles, or they can be damaged by the free radicals formed by ionization of cellular materials. The expression of chromosomal damage is unique to radiation, and to a few chemicals and drugs. Ordinarily, the chromosomes are very well insulated, each within it's own protective membrane, usually at or near the center of the cell. It is thus very difficult for most outside factors to damage them, without killing the cell first. So generally, effects which can damage the chromosomes make chromosome damage moot, since cell death would accompany or precede chromosome damage. At certain levels of exposure, this is not the case, with radiation poisoning. It is quite common for radiation exposure to damage the chromosomes, without causing immediate cell death. The traditional definitions, and symptoms of the illness stage are listed below.
Mild radiation sickness
Signs and symptoms resulting from an acute absorbed dose of
1 to 2 Gy may include:
-
Nausea and vomiting within 24 to 48 hours
-
Headache
-
Fatigue
-
Weakness
Moderate radiation sickness
With an acute absorbed dose of 2 to 3.5 Gy, a person may
experience:
-
Nausea and vomiting within 12 to 24 hours
-
Fever
-
Hair loss
-
Infections
-
Vomiting blood
-
Bloody stool
-
Poor wound healing
-
Any signs and symptoms associated with a lower absorbed dose
Moderate radiation sickness can be fatal to those most sensitive to radiation exposure.
Severe radiation sickness
An absorbed dose of 3.5 to 5.5 Gy can result in the
following signs and symptoms:
-
Nausea and vomiting less than one hour after exposure to radiation
-
Diarrhea
-
High fever
-
Any signs and symptoms associated with a lower absorbed dose
Severe radiation sickness is fatal about half the time.
Very severe radiation sickness
A person with an absorbed dose greater than 5.5 to 8 Gy can
have the following signs and symptoms:
-
Nausea and vomiting less than 30 minutes after exposure to radiation
-
Dizziness
-
Disorientation
-
Low blood pressure (hypotension)
-
Any signs and symptoms associated with a lower absorbed dose
The bodily system most vulnerable to radiation damage is the hemopoietic system, which uses cells in the bone marrow to produce red blood cells. Because the cells comprising this system are relatively unspecialized, have high metabolisms, and reproduce quickly, they are triply vulnerable, and act as a sort of a benchmark for radiation exposure. The first clinical symptom of radiation sickness is generally a lowering of the red blood cell count.
Other systems easily affected, due to the high reproductive rate of their component cells, are the reproductive, immune, and digestive systems. Victims of radiation syndrome are highly susceptible to infection, in addition to being anemic. Sterility (temporary or permanent) is also a common symptom. Victims of radiation syndrome will also generally exhibit signs of what appears to be sunburn. At high levels of exposure, the skin may blister and even char. At such high levels, the victim is as good as dead.
Up to a dose level of 500 - 1000 RADs, the major effects of the syndrome are caused by chromosome damage, and by the interference that this damage causes with the replication of DNA. At the 500 - 1000 RAD level, the lysosomes inside of the cell begin to burst. These organelles hold the various enzymes used for the digestive processes of the cell. Their rupture releases these substances, which then begin to dissolve cell structures. At around 3000 RADs, the mitochondria, the little power plants of the cell, stop working, and the cell starves. At 3000 to 5000 RADs, the cell membrane itself ruptures. This is kind of moot, since at any exposure in excess of 600 RAD, the patient will positively die without extensive medical attention. A victim with an exposure over 1000 RAD is considered to be unsaveable, no matter what treatment is given. Note the overlap with the instance of lysosome rupture.
Up to the 1000 RAD level, the most likely cause of death will be due to effects on the hemopoietic system, though infection due to impairment of the immune system adds a definite level of complication. Above 1000 RAD, the most likely cause of death will be due to failure of the GI tract. It is not that the hemopoietic effects go away, merely that the GI effects kill the victim more quickly than the hemopoietic effects otherwise would have. Death at this level of exposure, due to GI tract effects will occur within 3 to 5 days. There is no treatment.
The GI tract effects occur, because the cells lining the GI tract have an average lifespan of 1 - 3 days. These are expendable cells, which are shed and constantly replaced, sort of the like the outer layers of human skin. The problem here is that if production of these cells is interrupted, or halted, the body will run out, within 1 - 3 days, and the lower portions of the GI tract will be unlined. This will cause several very nasty things to happen. The victim will starve, of course, since nutrients can no longer be absorbed through the GI tract; but death will occur long before starvation. The GI tract is one of the dirtiest, most contaminated parts of the body. When the lining disappears, the huge, diverse bacterial and protozoac population of the tract has direct access to the body, and massive infection is the result. This is made even worse, by the fact that the immune system will also be greatly impaired at this level of exposure. In addition, the body is no longer able to absorb, or even to retain water, with the GI tract lining gone. So it becomes a race between infection, dehydration, and starvation, to see which kills the body first.
Interestingly, the nervous system, arguably the most complex, delicate, and generally most easily damaged system in the body, is one of the last systems to be affected by radiation exposure, probably due to the extremely low rate of reproduction among nerve cells, and their high rate of specialization. At one time, these cells were thought not to reproduce at all; but this has recently been found not to be true. Recent discoveries indicate that neurons in a certain central portion of the brain reproduce, and then migrate out to required areas, to settle in and send out connections to other cells. Still, this is an extraordinarily limited area, and for most of the brain, there is no reproduction of neurons. Radiation induced neural damage is generally caused by damage to the vascular system, leaking, and pressure, due to the destruction of blood vessels in the brain. If sufficient radiation has been absorbed to cause this condition, recovery is impossible. Death will occur within 30 hours of exposure.
Note that we are talking about ionizing radiation here, such as gamma rays, and X-rays. Neutrons are a whole different and more complicated story. Where ionizing radiation affects the electrons, neutrons affect the nucleus. A neutron can actually split atoms, or create radioactive isotopes from materials inside of the cell, essentially turning it in to a miniature nuclear reactor. In addition, an atom split by a neutron may give off further neutrons, though it is far more common for beta or gamma particles to be given off.
Treatment
At certain levels of exposure (up to about 500 - 1000 RADs), it is possible to treat affected persons, and to promote recovery. Above 1000 RADs, it is generally considered that nothing can be done, except to keep the patient as comfortable as possible until death. Below a dose of 300 RADs, it is likely that the victim will survive without intervention, though this is by no means certain. Below a dose of 200 RADs, there may be some latter complications; but the victim is almost certain to survive. Treatment may take several different forms.
Treatment for radiation poisoning is generally directed at stopping any further dosage from occurring. Cells that have already been damaged can not be assisted in repair. The best thing that can be done is to try and maintain the body as long as possible, to give it time to repair itself. Treating suppression of the immune system with antibiotics, trying to counter the red blood cell shortage with transfusions, and attempting to maintain the proper level of water in the body, are about all that can be done to counter death from radiation exposure. Available drugs are DTPA, which binds to metals and permits the body to rid itself of contaminants through urination, Prussian Blue, which does the same thing except that elimination is through defecation, and potassium iodide, which prevents radioiodine from entering the thyroid. Note that none of these "treatments" does anything about radiation already absorbed, but merely seeks to remove further sources of exposure. This can be useful in contaminated areas, if fallout is present; but otherwise does not do much good.