|
The Nuclear Tourist |
||
I am not a nuclear engineer, physicist, or technician, and am in no way officially connected with the nuclear industry. I do not hold myself up as any sort of expert on the subject; but have developed a keen interest in nuclear technology, and in the many ways that it might extend the survival of civilization. I have researched through books, the Internet, and occasionally by talking to guides, technicians, and even a scientist or two, at the various nuclear sites open to the public. I have gathered these researches together here, as a sort of a layman's guide to nuclear technology. What is most remarkable about this deep mysterious subject, is that it is not all that deep and mysterious, nor is it difficult to grasp the basics. There is no advanced math here, no deep physics, and nothing very abstract or confusing - this section was written so that even I can understand it. The reader who slogs all the way to the bottom of the page will know more about nuclear technology than most of the legislators, regulators, activists, and political hacks who presume to dictate how the industry should be run.
The discovery of nuclear fission, and then
fusion, seemed to hold out the Biblical prospect of both the blessing
and the malediction. Nuclear power was the answer to the world's energy
needs. Compared with coal, and oil, it was cheap, safe, and did not pollute.
These are virtues which it still has today. A pound of uranium has something
like the energy content of 3,000,000 tons of coal. It is actually cheaper
to produce power in a nuclear plant, than in a coal powered plant. When the
nuclear power generating industry first began, supporters predicted
energy that would be too cheap to meter. It is
also, despite the emotional rantings of the ignorant and fearful, quite a bit
safer. Far fewer people have been injured per kilowatt generated by
nuclear power, than is the case with coal powered plants. More people
have been killed in rail crossing accidents with trains transporting
coal to power stations, than have been killed in the entire history of
the nuclear program. Coal is also, like most minerals, slightly
radioactive. More radiation is put into the environment from the smoke
and gases generated by a coal powered plant, than is produced by any
nuclear plant - much more. Unfortunately, nuclear energy production has never
realized anything like it's full potential, in this country. It now
accounts for something like twenty percent of out total energy
production.
The anti nuclear, green, and liberal factions
are responsible for the stifling of the nuclear industry. The anti nuclear
faction portrays all nuclear plants as nuclear bombs ready to go off.
This is either a result of a complete lack of knowledge, or is an intentional
lie. A nuclear explosion is a particular kind of chain reaction which
is very artificial, and can only be produced intentionally under a specific
set of circumstances. Nuclear plants do not explode. As with many similar
movements, it gathered to itself large numbers of ignorant, fearful, backward
types. These are the same fear mongering types who, in the past, have burned
witches, called reading and math works of the devil, and forced doctors
of the past to go to grave robbers, so that they could study anatomy.
In addition to offering abundant energy, and
the world's ultimate weapon, the nuclear industry offers several different
types of medical technology. Nuclear isotopes are used to cure cancer,
as well as to treat certain other diseases. Nuclear agents are used as tracers
for metabolic processes, as well as markers for certain types of diagnostic
imaging. The use of radiological imaging dates back to the first X-ray
machines, which date back a hundred years or so. Research is still being
done; but radiation therapy; and imaging both promise to play more significant
roles in the future.
I begin to feel my age when I consider
this subject. For an increasing number of Americans today, the Cold
War is something out of history, and the Soviet Union is as dead a nation
to the younger generations, as Nazi Germany or the Ottoman Empire is to
my generation. While these days, terrorists, liberals, and apathy threaten to destroy
civilization, through the infliction of a thousand tiny cuts, there was
a time when the two superpowers threatened to destroy it, and mankind as
well, with one mighty blow. In the mean time, grim men were prepared to
destroy the world, in order to save civilization. Though this all sounds
very strange to those who did not live through the times, it was very real,
and very serious.
There is little doubt that nuclear power has more
than fulfilled it's military potential. Nuclear weapons have prevented
anything like a major war for decades, because they are so destructive
and horrifying that no one in their right mind would even consider
risking a nuclear war. Unfortunately, with the rise of the third world,
the leaders of many potential nuclear states are not in their right
minds. Nuclear power, on the other hand, is a different story.
The promise of nuclear power has barely been
acknowledged. much less fulfilled. Today something like a sixth of the
world's electricity comes from nuclear plants. Reserves of Uranium 235
are said to be enough to provide for the entire world's power needs, for a bit less than 100 years. Reserves of the much more
abundant Uranium 238 could last us for at least 10,000
years, and for as long as billions of years.
Even more promising are the huge amounts of thorium,
estimated to exist in triple the quantity of uranium. Imagine that; the world's energy needs for the rest of
conceivable history could be taken care of, had we but the will to use
the knowledge that we already have. Instead, in a hungry world, we
propose to burn food, in the form of corn derived ethanol,
for energy.
The Cast of Characters
The basic element (if you will pardon the pun) of the
modern nuclear industry is uranium. As an element, and a metal, Uranium has
a number of interesting properties. The metal is nearly as hard as steel,
and heavier than lead, being nearly as dense as gold. It tarnishes very easily,
and though it is a lustrous silvery metal, it will quickly turn black on
exposure to air. Powdered uranium may glow in the dark, will sporadically
burst into flames, and can even react with water, in the manner of sodium.
It is pretty strange stuff. One of the more interesting characteristics,
of Uranium, as well as most other unstable elements, is that it is slightly
warm to the touch.
Though it's compounds had been used since Roman times,
uranium was not properly discovered until 1789, and was not isolated until 1841. In 1896,
uranium was discovered to be radioactive, which accounts for it's warmth.
Though considered to be an exotic material, by most people,
Uranium is quite common, being a bit more abundant than tin.
It is also relatively cheap; cheap enough to have been used
as counterweights in aircraft. Depleted, and even natural,
uranium can be found for sale, here and there. To the right is a photograph of a
sample for sale at United Nuclear. Uranium is also commonly used in many other
non nuclear roles, such as weights in gyro
systems, and in a number of other applications where a durable, and heavy
substance is required. The metal itself is rarely used in reactor applications.
Generally, reactor fuel consists of a number of pellets of the oxide, encased in
a series of long tubes.
was discovered to be radioactive, which accounts for it's warmth.
Though considered to be an exotic material, by most people,
Uranium is quite common, being a bit more abundant than tin.
It is also relatively cheap; cheap enough to have been used
as counterweights in aircraft. Depleted, and even natural,
uranium can be found for sale, here and there. To the right is a photograph of a
sample for sale at United Nuclear. Uranium is also commonly used in many other
non nuclear roles, such as weights in gyro
systems, and in a number of other applications where a durable, and heavy
substance is required. The metal itself is rarely used in reactor applications.
Generally, reactor fuel consists of a number of pellets of the oxide, encased in
a series of long tubes.
There are some hazards associated with common uranium, however, they
are often overstated. The metal is only weakly radioactive,
in it's natural state, and does little or no harm unless it is ingested. Ingested
uranium is more of a threat due to heavy metal poisoning,
than to any radiation danger. The most common cause of
death, from uranium, is from kidney failure, due to heavy
metal poisoning. Interestingly, the most hazardous by product of
the whole nuclear process is not nuclear waste. It is a series of fluoride compounds
left over from the refining process.
Nuclear technology is like any
other; there is a learning curve, and there are hazards. The
users of the first wheels, undoubtedly went too fast, and
occasionally went out of control, as do modern users of
wheels. Early man certainly got burned, when he first began
to use fire, and doubtless drowned soon after the launching
of the first boat. Everything has a cost; but
there is also a cost to not using things. Like it or not,
and whether we knew what we were doing or not, mankind has
brought itself into the nuclear era.
The various isotopes of uranium, are examples of the two types of major nuclear materials. The first type is the fissionable material, which is what most people think of, when they think of nuclear materials. The fissionable natural isotope of uranium is 235. This is the type of material that will split to create energy, and will have a critical mass. The critical mass of a fissionable is the weight of the substance which, when formed into a sphere, would support a chain reaction. There are ways to initiate chain reactions with less material, either through compression, or through the use of outside sources of radiation; but the critical mass of a given fissionable, is a pretty good guide as to how usable it would be as an explosive, or as a source of energy.
Generally, a fissile material will decay at a certain rate, defined by its half life. The half life of a material is the amount of time that it would take for half of a given sample to decay. As an example, with a half life of a bit more than 24,000 years, a one pound sample of plutonium 239, would, after 24, 000 years, only contain a half pound of P239, the rest having transmuted into U235, through alpha decay. This decay is spontaneous, and apparently random, and has nothing to do neutron inspired fission. Once critical mass is attained, things are different. Neutrons are given off as part of the normal decay process, but generally have no effect on the surrounding atoms. Because these neutrons are fast moving, and have no charge, they can pass by, and even pass through, other atoms without affecting them, and simply pass out of the mass of fissionable which produced them. As the mass gets larger, there is more material to pass through, and the likelihood of a strike increases. Eventually, there is a point at which there is so much material, that a strike is virtually certain. This strike will split a nucleus, and cause it to release further neutrons, which will strike other nuclei and cause even more neutrons to be released, to strike other nuclei, and a chain reaction occurs. This considerably speeds up and changes the nature of the transmutation of the mass. Instead of years, centuries, or millennia, we are talking microseconds. We are also talking a change in the materials produced. Rather than giving off alpha particles, as is the case with natural nuclear decay, neutron struck atoms tend to cleave more nearly in half. The critical mass is determined by the density, stability, and neutron cross section of the element in question. Some fissionables, like U238, are so stable, that they have no critical mass.
The most commonly used fissionable materials are uranium 235, and plutonium 239. The element numbers indicate the isotope, by enumerating the atomic weight. The atomic weight of an element is essentially the total number of neutrons and protons. Thus uranium, with an atomic number of 92 (92 protons) with have 143 neutrons in isotope 235, and 146 neutrons for isotope 238. Uranium 235, and plutonium 239 are the most commonly used, because they are unstable enough to have a critical mass. These materials will spontaneously create, and sustain chain reactions, when critical mass is accumulated. Thus a large enough chunk of plutonium will immediately begin to produce significant amounts of heat, as well as radiation. There is something almost frighteningly simple about this. You can take two small pieces of plutonium, and they will be somewhat warm to the touch. Slap them together, and they will immediately grow hot enough to boil water, or char your hands.
Natural uranium does not have much of the 235 isotope. As a matter of fact, the 235 isotope is very nearly at trace element levels, comprising only about 0.7% of a refined sample of naturally occurring uranium. This is pure metallic uranium, refined from ore, and separated out, just as iron or gold might be separated out into their pure metallic forms. Still, for most uses, the pure uranium must further be separated into its various isotopes. The process is generally done by weight, and is known as enrichment. Enriched uranium is higher in U235 than natural uranium. The degree of enrichment depends upon the intended use of the material. In addition to the enriched material, there is a residue, which is virtually pure U238. This is commonly known as depleted uranium, because the valuable, and sought after U235 has been taken out.
The second type of nuclear material is the fertile material. Fertile nuclear material is generally not usable as a source of energy; but may be converted into something that is. A good example of this is uranium 238. This material, though slightly radioactive, can not be made to support a chain reaction, and has no critical mass. When exposed to radiation, as in the walls of a reactor, and struck by neutrons, uranium 238 is converted into uranium 239, which quickly decays into neptunium 239. With a half life of around 56 hours, neptunium 239 quickly decays into plutonium 239. Thus Uranium 238 is the major source of plutonium 239. A major fertile metal is thorium, which is abundant, and easily converted.
There are many other useful radioactive materials; but they are pretty much limited to the role of supporting players for the main characters. Some are good neutron sources, while others are neutron reflectors. A list of all of the major nuclear materials is below, in table form. There are a number of other fissile, and fusile materials; but most of these are either too dangerous, or in too short supply to be considered useful.
| Fissile Materials | |||||||
| Substance | Protons/Neutrons | Decay | Half life | Critical sphere | Density | Curies | |
| Plutonium 239 | 94/145 | alpha | 24,110 years | 22 pounds | 19.8 gm cm | .063 gm | This is the most common material for use in nuclear warheads |
| Uranium 236 | 92/144 | alpha | 23,420,000 years | 18.9 gm cm | .00006 gm | A neutron poison, and waste product in thermal reactors, it is also quite radioactive. | |
| Uranium 235 | 92/143 | alpha | 704,000,000 years | 115 pounds | 18.9 gm cm | .0000021 gm | This is the most common material for use in nuclear reactors. |
| Uranium 233 | 92/141 | alpha | 159,200 years | 35 pounds | 18.9 gm cm | .0095 gm | Not presently used; but has great potential |
| Fertile Materials | |||||||
| Substance | P/N | Decay | Half life | Critical | Density | Curies | |
| Thorium 232 | 90/142 | spontaneous fission |
14,100,000,000 years | none | 11.724 gm cm | .00000011 gm | Could be a major source of uranium 233 |
| Uranium 238 | 92/146 | alpha | 4,460,000,000 years | none | 18.95 gm cm | .00000033 gm | The major source of plutonium 239 |
| Fussile Materials | ||||||
| Substance | Decay | Half life | Critical | Density | Curies | |
| Lithium 6 | NA | NA | NA | .5 gm cm | NA | |
| Lithium 7 | NA | NA | NA | .534 gm cm | NA | |
| Deuterium | NA | NA | NA | gas | NA | |
| Tritium | beta | 12.32 years | NA | gas | 9650 gm | Though it can be used in a fusion device, the most common uses for tritium are either as a trigger neutron source, or as a booster for fission bombs. |
| Other Materials | ||||||
| Substance | Decay | Half life | Critical | Density | Curies | |
| Polonium | alpha | 138.4 days | 9.5 gm cm | 4500 | A very powerful source of alpha particles, for nuclear triggers. | |
| Beryllium | NA | NA | NA | NA | A good neutron reflector. Also a good source of neutrons, when combined with an alpha emitter. | |
| Cadmium | NA | NA | NA | NA | A neutron absorber | |
| Boron | NA | NA | NA | NA | A nuclear poison | |
| Graphite | NA | NA | NA | NA | Used as a moderator to slow down fast neutrons. | |
| Radium 226 | alpha | 1600 years | 5.5 gm cm | 1 | ||
The chart above, though a useful guide, is only somewhat accurate. This is due to the changing nature of radioactive elements, and to their changing densities, and varying states. Hot uranium expands, and greatly reduces it's density, over that of cold uranium. These substances are always changing, and thus the nuclear properties of any given amount of any such substance are dynamic, rather than static. As an example, a sample of tritium loses half of it's radioactivity, every twelve years or so, as it decays into helium. In contrast, the radioactivity of a sample of uranium 235 increases greatly, as it decays into thorium 231, which is around 2.6 x 10^11 more radioactive, and as the amounts of uranium 236 increase. So suddenly, after a few years, your sample of mildly radioactive uranium 235, is an extremely radioactive sample of uranium 236, thorium, protactinium, and a few other unhealthy substances. This also happens, though to a lesser extent, with the far more common uranium 238.
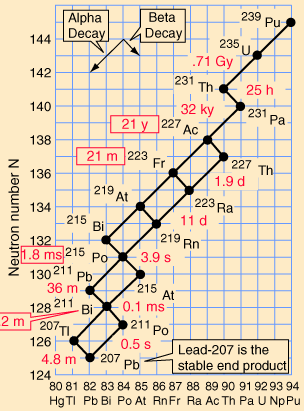
Decay series
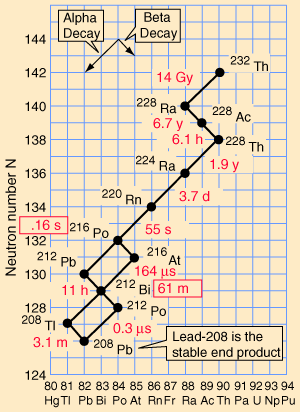 Eventually, all of the fissile
elements end up as stable elements, at the end of what are generally
called their
Eventually, all of the fissile
elements end up as stable elements, at the end of what are generally
called their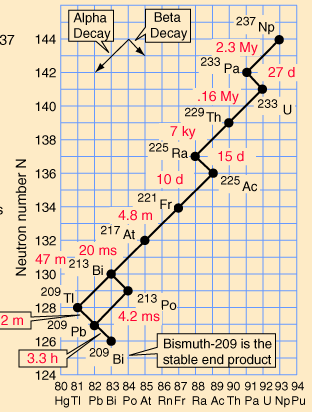 decay series',
all having found their lowest levels, as it were. It might seem that there should be a variety
of different elements into which the fissiles can transform themselves,
and indeed, there are a number of intermediate fissile elements in each
series; but in the end all are transmuted into one of only four stable
elements. These are known as the four natural decay series. I have
included a great set of charts, taken from a physics website, showing
the members of these four series, which together include all naturally
occurring radioactive elements. These charts show how the decay
progresses in each series. Though it is possible to create other
radioisotopes, outside of what is show in these charts (strontium 90,
cobalt 60, and many others), these are not natural decay products, and
are made by bombarding elements with neutrons to take them up, and
increase their atomic weight or number, whereas the natural decay
series' always travel in a downward direction, toward lower atomic
numbers, and lower atomic weights.
decay series',
all having found their lowest levels, as it were. It might seem that there should be a variety
of different elements into which the fissiles can transform themselves,
and indeed, there are a number of intermediate fissile elements in each
series; but in the end all are transmuted into one of only four stable
elements. These are known as the four natural decay series. I have
included a great set of charts, taken from a physics website, showing
the members of these four series, which together include all naturally
occurring radioactive elements. These charts show how the decay
progresses in each series. Though it is possible to create other
radioisotopes, outside of what is show in these charts (strontium 90,
cobalt 60, and many others), these are not natural decay products, and
are made by bombarding elements with neutrons to take them up, and
increase their atomic weight or number, whereas the natural decay
series' always travel in a downward direction, toward lower atomic
numbers, and lower atomic weights.
These series' end with the stable elements having atomic weights of 206, 207, 208, and 209. These are all isotopes of lead, except for 209, which is bismuth. The reason that there are four, and only four decay series, is due to the nature of nuclear decay. There are essentially two ways that a nucleus can decay. There is alpha decay, and beta decay. The reasons for this are known, to a degree; but are beyond the scope of this page. Because these are the only two natural ways for radioactive elements to decay and transmute, the limit of four distinct series is a given, with the number of series defined by the size of the alpha particle, which itself consists of four nuclear particles.
 Of the two types of natural decay, beta decay makes no change in atomic
weight; but does change the
Of the two types of natural decay, beta decay makes no change in atomic
weight; but does change the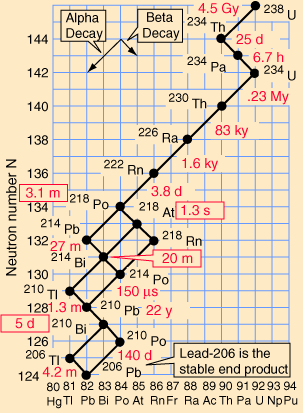 atomic number. Such changes are known as transmutations, and change one
element into another element. Thus, when tritium, an isotope of
hydrogen, beta decays, it transmutes into helium-3, a light helium
isotope. The atomic weight
remains approximately the same; but the atom itself is transmuted from
element number one (hydrogen) into element number two (helium). In this
case, the change occurs because one of the three neutrons in the tritium
atom, decays into a proton. In doing so, this neutron gives off a beta
particle, which is essentially a high speed electron. To be even more
explicit, one of the down quarks in the neutron beta decays, giving off
a beta particle, and an antineutrino. Because a neutron is composed of two down
quarks, and one up quark, and a proton is composed of one down quark and
two up quarks, this has the effect of changing a neutron into a proton.
atomic number. Such changes are known as transmutations, and change one
element into another element. Thus, when tritium, an isotope of
hydrogen, beta decays, it transmutes into helium-3, a light helium
isotope. The atomic weight
remains approximately the same; but the atom itself is transmuted from
element number one (hydrogen) into element number two (helium). In this
case, the change occurs because one of the three neutrons in the tritium
atom, decays into a proton. In doing so, this neutron gives off a beta
particle, which is essentially a high speed electron. To be even more
explicit, one of the down quarks in the neutron beta decays, giving off
a beta particle, and an antineutrino. Because a neutron is composed of two down
quarks, and one up quark, and a proton is composed of one down quark and
two up quarks, this has the effect of changing a neutron into a proton.
Alpha decay, as can be seen in the element chart above, is far more common. Alpha decay also transmutes the element from which it is produced; but unlike beta decay, alpha decay also changes the atomic weight of the element. Alpha decay occurs, when the atoms of an element give off alpha particles. An alpha particle is essentially a helium nucleus, consisting of a pair of neutrons, and a pair of protons.
You might think that other types of decay would be possible; but under normal circumstances, they are not. There is no proton decay, nor do fissile nuclei commonly give off neutron/proton pairs or single neutrons, mostly due to energy thresholds required for particles to escape the nucleus. These can all be made to happen, in accelerators and other such places; but do not generally occur in nature. So every naturally occurring radioactive substance will appear on one of the four charts above, and all will eventually end up as one of the stable elements shown at the bottom of the charts. Thus the four natural decay series' take their forms. In every one of these reactions, the nucleus is attempting to get to the lowest possible energy state.
Energy Potentials
Fission of U-233: 17.8 kt/kg
Fission of U-235: 17.6 kt/kg
Fission of Pu-239: 17.3 kt/kg
Fusion of pure deuterium: 82.2 kt/kg
Fusion of tritium and deuterium (50/50): 80.4 kt/kg
Fusion of lithium-6 deuteride: 64.0 kt/kg
Fusion of lithium-7 deuteride
Total conversion of matter to energy: 21.47 Mt/kg
1 kt. fission of 0.241 moles of material (1.45x10^23 nuclei)
fission of approx. 57 g of material
1.16x10^6 kilowatt-hrs
We are in the midst of
the nuclear era, though this is a fact that we scarcely seem
to notice. The end is nowhere in sight, despite the efforts
of the anti nuclear activists. There is no end in sight for
the simple reason that we are now dependant on nuclear
technology, in a number of different ways. Everyone is
pretty much aware of the use of nuclear weapons, and nuclear
power generation, and is somewhat aware of the use of
radioactive materials in medicine; but there are scores of
other uses. Nuclear materials are used in smoke detectors,
food processing and sterilization, materials testing, and a
number of manufacturing processes.
Something like 7% of the worlds energy is derived from
nuclear power, while in the
Regarding nuclear weapons we have kind of got the wolf by the
ears with this one. We are not necessarily happy about the
situation; but we don't dare let go. If
So we are in the midst of the nuclear era, and see no end in
sight; but what about the beginning? Well that's a bit
harder to determine, and is as much a matter of judgment as
anything else. Some might say that the nuclear era began
with the discovery of X-rays, or the discovery of
radioactivity, both made around the turn of the last
century. For others, it might seem that the splitting of the
atom, first accomplished on
judgment as
anything else. Some might say that the nuclear era began
with the discovery of X-rays, or the discovery of
radioactivity, both made around the turn of the last
century. For others, it might seem that the splitting of the
atom, first accomplished on
Still, all of
these events were mere stepping stones --- experiments which
produced no usable results, and had no effect on the larger
world, outside of the laboratory. These are all examples of
science, of exploration and proof. An era does not start
until the engineers get involved, and create devices which
take the discoveries of the scientists out of the
laboratory, and put them into the world of common
experience. This did not happen until the forties. On
For myself, as for most others, the nuclear era began
on